Canada’s West Coast on-the-water choice for over 60 years. We have two of the finest West Coast locations to serve you, as well as the highest standards in yacht brokerage.


Thunderbird Marina
West Vancouver, BC

Yacht Sales

Westport Marina

THUNDERBIRD MARINE
Our locations are key.
From Victoria, Vancouver, and the Gulf Islands to the Sunshine Coast and Desolation Sound, Thunderbird is a name you can count on to make the most of your on-the-water experience.

YACHT SALES AND SERVICES
Not only do we offer two fabulously located marinas to moor your boat, we also provide a wide selection of services to help you maintain, buy or sell your boat in Vancouver or on Vancouver Island.

28′ Bayliner 2855 Ciera

28′ Maxum 2800 SCR

42′ Boathouse
West Vancouver

38′ Bayliner 3818

24′ Sea Ray 240 Sundancer

46′ C&L Marine Corp 46 Trawler
thunderbird marina
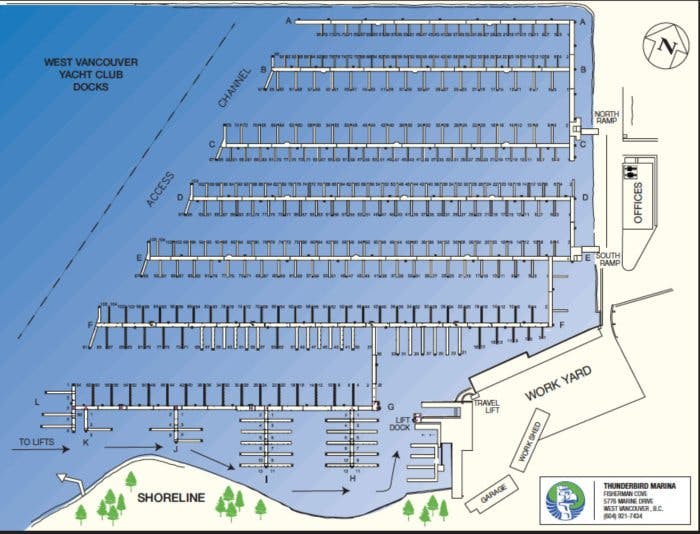
west port marina
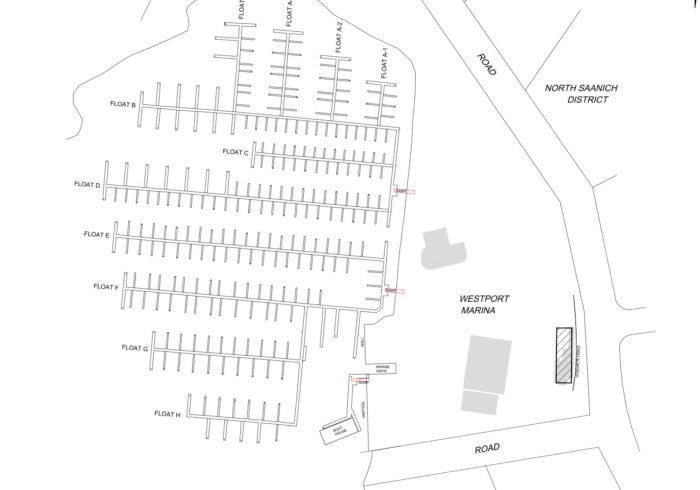
Vancouver Yacht Sales
SIDNEY YACHT SALES
PORT MOODY YACHT SALES
Thunderbird Yacht Sales
Photos & videos, location & hours.
Suggest an edit
5776 Marine Drive
West Vancouver, BC V7W 2S2
Recommended Reviews
- 1 star rating Not good
- 2 star rating Could’ve been better
- 3 star rating OK
- 4 star rating Good
- 5 star rating Great
Select your rating
Overall rating

What an exceptionally wonderful experience dealing with Sonia and the other sales staff at this Thunderbird location. Incredibly, my sailboat sold within a week, and for more than I originally paid for her just a few years earlier! Sonia was incredible, as she was happy to come to my location on Bowen Island to perform all the preliminaries before posting the boat for sale. Prior to contacting Thunderbird, I had tried to sell my sailboat on Craigslist, and for an entire year, I wasted up to an hour on each whinging tire-kicker who actually had zero interest on following through on a purchase. The Craigslist Hall of Fame: - Wanted a $10K reduction in price, as they weren't pleased with the Yanmar 2GM. - The American who wanted me to sail the boat to Seattle for him...for free. -The couple who offered chump change, and also wanted me to sail the boat for them to Shelter Island. -The 'Hard-of-Thinking' time waster who contacted me on multiple occasions, not realizing that he had already seen the boat. Working through Sonia, we avoided all this horrific nonsense, and as mentioned, the boat sold in under a week!
Thunderbird Yacht Sales provides a professional brokerage service to sell your boat, You will be treated with respect and probably also get the best price that can be attained for your boat. Cormac and Andy worked diligently and it resulted in the sale of Bel-Air at a very fair price.
I was into the Reed Point Location today to look at boat inventory as I am in the market. The arrogance of the sales associate at that location had me walk out with no interest in spending my money there. Fortunately there are many other places to shop for a boat.
1 other review that is not currently recommended
- fr Passer en français / Switch to French language
- 604-921-7457 Primary
- 604-921-7486 Fax
- www.thunderbirdmarine.com

Thunderbird Yacht Sales
- Like this business? Add a photo
Thunderbird Marine Corp. has provided turn-key moorage, boatyard and sales service to sailing and powerboat enthusiasts on Canada's West Coast for over 60 years. From Victoria, Vancouver, and the Gulf Islands to the Sunshine Coast and Desolation Sound, Thunderbird is a name you can count on to make the most of your on-the-water experience. We offer 3 fabulously located marinas to house your boat, as well as a complete complement of services to help you maintain, buy or sell your used boat in Vancouver or on Vancouver Island. more... See more text

Opening Hours *Holiday hours
| Monday | |
|---|---|
| Tuesday | |
| Wednesday | |
| Thursday | |
| Friday | |
| Saturday | |
| Sunday |
| Verify with Merchant |
|---|
Social Media
- Write a review
- Suggest an update
Ratings & Reviews - Thunderbird Yacht Sales
Get an opinion about this business.
Think your friends might be familiar with this business? Ask your friends on Facebook to see what they think.
- Marinas in BC |
- Marinas in West Vancouver |
- Marinas in Caulfeild |
- Boat Dealers & Brokers |
- Boat Dealers & Brokers in BC |
- Boat Dealers & Brokers in West Vancouver |
- Boat Dealers & Brokers in Caulfeild |
- North & West Vancouver |
- People search
- Get a free listing
- Advertise with us
- Download the app
- About Thunderbird
- Our Moorage Rates
- Thunderbird Services
- Service Partners
- Yacht Sales
Welcome to Thunderbird Marina
The Ultimate West Vancouver Marine Experience at Fisherman’s Cove.
Vancouver’s Number One Choice
Your full-service marina, the latest news & announcements, meet evolutions marine.
posted 10/20/2023
Thunderbird Marina is pleased to welcome Evolutions Marine to the family of onsite marine professionals. Having owned and...
FALL INTO WINTER STORAGE
posted 10/15/2023
If you have been to the marina recently you will notice that the parking lots are filling with...
Map & Location of Our Facilities
Quietly nestled in Fisherman’s Cove in West Vancouver BC, our map reveals why our location can’t be beat. With over 600 slips available, every location is a good location!
Fisherman’s Cove
Directions to Boat Yard
Get In Touch With Us
Thunderbird Marina offers moorage, storage, haulout facilities, and shops for your boating needs. Contact us for more information.
Contact Thunderbird now

Thunderbird Marine Locations
Thunderbird Marina in West Vancouver
Yacht sales port moody at reed point marina, westport yacht sales, yacht sales west vancouver at thunderbird marina, westport marina sidney on vancouver island, top categories.
- + Accommodations
- + Boat Brokers / ...
- + Boat Builders
- + Canvas / Boat Tops
- + Charters / Boat...
- + Charts / Maps
- + Electronics / C...
- + Engines / Outbo...
- + Financial
- + Insurance
- + Maintenance / R...
- + Marine Hardware...
- + Real Estate
- + Restaurants / Pubs
- + Sailboat Riggin...
- + Yacht Clubs / A...
- Quadrant Marine Institute
- Port Alice Rumble Beach Marina
- Stem To Stern Marine Service
- Boating BC Association
- The Waterfront Suites & Marina
- Nootka Sound Resort

Thunderbird Yacht Sales
Contact and address.
| Address: | 5776 Marine Dr, West Vancouver, BC V7W 2S2, Canada |
|---|---|
| Postal code: | V7W 2S2 |
| Phone: | (604) 921-7457 |
| Website: |
Opening Hours:
| Monday: | 9:00 AM – 5:00 PM |
|---|---|
| Tuesday: | 9:00 AM – 5:00 PM |
| Wednesday: | 9:00 AM – 5:00 PM |
| Thursday: | 9:00 AM – 5:00 PM |
| Friday: | 9:00 AM – 5:00 PM |
| Saturday: | 9:00 AM – 5:00 PM |
| Sunday: | 9:00 AM – 5:00 PM |
Location & routing
Bought a boat in 2min
Great service from Sonia, excellent communication and professionalism!
Sonia is an amazing person. Utterly amazing. In terms of sales, and sheer professionalism, I have yet to meet anyone else of her calibre. We brought our sailboat to her to sell, and with her incredible talent, the boat was sold within a week, and actually sold for more than its purchase price two years earlier! This is a feat of superhuman ability!
Cormac iz the guy to see for knowledge on boats
Photos of Thunderbird Yacht Sales

SIMILAR IN THE AREA
- Vancouver Outboard Centre Ltd 5776 Marine Dr, West Vancouver, BC V7W 2S2, Canada Point of interest | Establishment
- BC Boat Clean 5776 Marine Dr, West Vancouver, BC V7W 2S2, Canada Point of interest | Establishment
- Thunderbird Marina 5776 Marine Dr, West Vancouver, BC V7W 2S2, Canada Point of interest | Establishment
- Performance Yacht & Indl Svc 5776 Marine Dr, West Vancouver, BC V7W 2S2, Canada Point of interest | Establishment
- Exo Projects 5800 Marine Dr, West Vancouver, BC V7W 2S2, Canada Point of interest | Establishment | General contractor
- BC Electrical Services Ltd 5747 Telegraph Trail, West Vancouver, BC V7W 1S4, Canada Electrician | Point of interest | Establishment
- Eagle Harbour Holdings 5758 Larson Pl, West Vancouver, BC V7W 1S4, Canada Point of interest | Establishment
- Future Flow Media 5775 Marine Dr, West Vancouver, BC V7W 2S1, Canada Point of interest | Establishment
- Marina Bay Academy Educational Childcare 5775 Marine Dr, West Vancouver, BC V7W 2S1, Canada Point of interest | Establishment
- Envisioning + Storytelling 5775 Marine Dr, West Vancouver, BC V7W 2S1, Canada Point of interest | Establishment
Most Recent
- Bright Edge Dentistry [Uncategorized]
- Etobicoke Martial Arts [School]
- The Concrete King of Burnaby [General contractor]
- Home Painters Pro [Painter]
- Premier Loans Canada [Finance]
- Technocrats Digimate [Uncategorized]
- MAXUM Fitness [Gym]
- Active Physiotherapy Brampton [Physiotherapist]
- Dental Care Centre [Dentist]
- Land Mark Repair [Car repair]
Today most viewed
- Meewasin Park
- Cougar Canyon Ecological Reserve
- Premier Envelope (B C) Ltd
- The Pureland Health Clinic
- LANVis Corporation
- Industries Renaud Gravel Inc (Les)
- Tomanek Farms Ltd
- BOSS Supplements
- Quality Architect Firm
- Active Physiotherapy Brampton
Home page . + Add listing . About . Privacy Policy . Terms of Service . Contact Us .
© 2024 CanadaVerified.info All Rights reserved.
Thunderbird Marina
Photos & videos, services offered.
Verified by Business
Boat repair
Boat dealers
You Might Also Consider

Ocean Trailer Rentals
31.4 km away from Thunderbird Marina
Ocean Trailer is Western Canada's Trailer Specialist. We sell, buy, lease, rent, service commercial trailers and sell trailer parts. We have mobile mechanic service, and a full service trailer wash bay. read more
in Trailer Rental, Trailer Dealers, Trailer Repair

No. 1 Collision Group
20.2 km away from Thunderbird Marina
Daniel T. said "I went to this bodyshop a couple weeks ago to get my car repaired after an accident. The entire process of handing over my car keys to getting my car back and repaired took one week, which was very reasonable considering they had to…" read more
in Auto Detailing, Body Shops
Location & Hours
Suggest an edit
5776 Marine Drive
West Vancouver, BC V7W 2S2
Recommended Reviews
- 1 star rating Not good
- 2 star rating Could’ve been better
- 3 star rating OK
- 4 star rating Good
- 5 star rating Great
Select your rating
Overall rating
Thunderbird marina. Sales people are very arrogant and unprofessional. If they don't like what you say, they tried to convince you to do something that goes totally against your wishes and keep saying what is such a good deal yet they have no idea terrible company to deal with this all I can say.

I have moored my yacht at Thunderbird for many years. I have always found them honest and reliable. The marina is clean and well maintained and facilities are excellent, among the best in the Vancouver area. It isn't cheap, but I believe they give fair value.
Be very careful when dealing with this company. We just spent over $1,000 and two days of our time to find out that the listing was incorrect, the boat was listed as a 1988 and our survey proved it was a 1981. In double checking the listing on Yacht World (after the fact) it was listed correctly with the sales person's name on the bottom that we actually dealt with, indicating that all is not as it appears, when we questioned the sales staff and even the owner was not surprised with the age difference. We would caution anyone wanting to do business with these folks to do so carefully.
Other Places Nearby
Find more Boat Dealers near Thunderbird Marina
Find more Boat Repair near Thunderbird Marina
Related Cost Guides
Advertising
Career Counseling
Editorial Services
Graphic Design
Music Production Services
Private Investigation
Shredding Services
Car Brokers
Car Dealers
Gas Stations
Motorsport Vehicle Repairs
Oil Change Stations
Transmission Repair
People Also Viewed

Marisol Marine Center
Stem To Stern Marine Service

Bridgeview Marine

Inletmarine
Vancouver Small Outboards
MK Procraft Jet Ski ATV & Sled Repair
Windward Yacht Sales

Lynnwood Marina & Boatyard
Haruna Sales & Service

Sunset Marina
- British Columbia
- West Vancouver
- 5776 Marine Dr
Thunderbird Marina
Thunderbird Marine Corp. has provided turn-key moorage, boatyard and sales service to sailing and powerboat enthusiasts on Canada's West Coast for over 60 years. From Victoria, Vancouver, and the Gulf Islands to the Sunshine Coast and Desolation Sound, Thunderbird is a name you can count on to make the most of your on-the-water experience. We offer 3 fabulously located marinas to house your boat, as well as a complete complement of services to help you maintain, buy or sell your used boat in Vancouver or on Vancouver Island.
Thunderbird Marina - 5776 Marine Dr, West Vancouver, BC
Located at 5776 Marine Dr near you, Thunderbird Marina is a local business within the marinas category of Canpages website.
Phone 604-921-7434 to get in contact with Thunderbird Marina that is located in your neighbourhood.
Finally, you can send this to your friends by clicking on Facebook or Twitter links.
Opening Hours
Monday 9:00 am - 5:00 pm
Tuesday 9:00 am - 5:00 pm
Wednesday 9:00 am - 5:00 pm
Thursday 9:00 am - 5:00 pm
Friday 9:00 am - 5:00 pm
Saturday 9:00 am - 5:00 pm
Sunday 9:00 am - 5:00 pm
- Boat Repair
- Boat Equipment & Supplies - Retail
- Storage - Boat & Recreational Vehicles
- Marine Repairs
- Scarborough
- Mississauga
- Quebec City
- Pet Grooming
- Tanning Salons
- Fitness Centers
- Restaurants
- Car Repairs
- Electricians
- Beauty Salons
- Chiropractors
- Yacht Listings
- Buyer Seller Resources
- Meet the Team
- Why Use a Yacht Broker?
Thunderbird Yacht Sales
West Vancouver at Thunderbird Marina (604) 921-7457 Port Moody at Reed Point Marina (604) 939-0499 Vancouver Island at Westport Marina (250) 656-5832
Boats & Yachts for sale
- Listing No
- Thumbnails
Manufacturer
List your boat.
Interested in listing your boat with us?

IMAGES
COMMENTS
Welcome to THUNDERBIRD YACHT SALES. ... 5776 Marine Drive West Vancouver, British Columbia Canada V7W 2S2 +1 (604) 921 7457 +1 (604) 921 7486. [email protected]. Sidney Yacht Sales @ Westport Marina 2075 Tryon Road Sidney, British Columbia Canada V8L 3X9 +1 (250) 656 5832
Thunderbird Yacht Sales. West Vancouver at Thunderbird Marina (604) 921-7457 Port Moody at Reed Point Marina (604) 939-0499 ... 5776 Marine Drive West Vancouver, British Columbia Canada V7W 2S2 +1 (604) 921 7457 +1 (604) 921 7486. [email protected]. Sidney Yacht Sales
Thunderbird Marine has provided premier moorage, boatyard and sales service to sailing and powerboat enthusiasts on Canada's West Coast for over 60 years. ... Vancouver Yacht Sales @ Thunderbird Marina 5776 Marine Drive West Vancouver, British Columbia Canada V7W 2S2 +1 (604) 921 7457 +1 (604) 921 7486 [email protected]. SIDNEY ...
Thunderbird Marina, West Vancouver, British Columbia, V7W 2S2, Canada. Thunderbird Marine Corp. is perhaps the most established of any boating company in Western Canada. Founded in 1952 Thunderbird Yacht Sales has full service yacht brokers and associate broker centres. Clear Filter Owner: broker-thunderbird-yacht-sales-15619.
Thunderbird Yacht Sales. West Vancouver at Thunderbird Marina (604) 921-7457 Port Moody at Reed Point Marina (604) 939-0499 ... 5776 Marine Drive West Vancouver, British Columbia Canada V7W 2S2 +1 (604) 921 7457 +1 (604) 921 7486. [email protected]. Sidney Yacht Sales
Thunderbird Marina. West Vancouver, BC. Yacht Sales. Visit. ... Sidney, BC. About. THUNDERBIRD MARINE. Thunderbird Marine has provided premier moorage, boatyard and sales service to sailing and powerboat enthusiasts on Canada's West Coast for over 60 years. OUR LOCATIONS ARE KEY ... Latest Boats & Yachts For Sale. 36′ Tiara 36 Convertible ...
2 reviews of THUNDERBIRD YACHT SALES "What an exceptionally wonderful experience dealing with Sonia and the other sales staff at this Thunderbird location. Incredibly, my sailboat sold within a week, and for more than I originally paid for her just a few years earlier! Sonia was incredible, as she was happy to come to my location on Bowen Island to perform all the preliminaries before posting ...
West Vancouver. Region: British Columbia. Distance: 3552 km. Phone: 1-877-926-2822 Enter ext: 7647. The Thunderbird Marina story begins in 1952 with a small marina opening to service local boaters in Fisherman's Cove, West Vancouver. With a protected location, stable docks and exemplary service, our business quickly grew, and by 1970 we had ...
Thunderbird Yacht Sales. Phone Number. 604-921-7457 Primary; 604-921-7486 Fax; Directions. Website. www.thunderbirdmarine.com; Opening soon 9:00 am, See all hours. Thunderbird Yacht Sales. 5776 Marine Dr, West Vancouver, BC V7W 2S2 Get directions ...
West Vancouver Yacht Sales. Thunderbird Marina 5776 Marine Drive West Vancouver, British Columbia Canada V7W 2S2. Phone: +1 (604) 921 7457 Email: [email protected]. Sidney Yacht Sales. Westport Marina 2075 Tryon Road Sidney, British Columbia Canada V8L 3X9. Phone: (250) 656 5832
MEET EVOLUTIONS MARINE. posted 10/20/2023. Thunderbird Marina is pleased to welcome Evolutions Marine to the family of onsite marine professionals. ... 5776 Marine Drive West Vancouver, British Columbia Canada V7W 2S2 +1 (604) 921 7434 ... (604) 921 7486 [email protected] For Yacht Sales & Service. Visit Thunderbirdmarine.com ©2024 Thunderbird ...
Thunderbird Marina, West Vancouver, British Columbia, V7W 2S2, Canada. Thunderbird Marine Corp. is perhaps the most established of any boating company in Western Canada. Founded in 1952 Thunderbird Yacht Sales has full service yacht brokers and associate broker centres. Clear Filter Owner: broker-thunderbird-yacht-sales-15619.
5776 Marine Drive West Vancouver, BC V7W 2S2. Fax: 604.921.7486. 604.921.7457. Map. E-mail. Business Details; Share by Email Tweet. About Us. On just about any day of the year Thunderbird Yacht Sales can have as many as 100 boats or more on display ashore and afloat adjacent to its' waterfront offices. Check out our inventory on-line and swing ...
Yacht Sales West Vancouver at Thunderbird Marina 5776 Marine Drive West Vancouver, BC V7W 2S2 Ph: 604.921.7457 Fax: 604.921.7486 E-mail | Hours. 5. Westport Marina Sidney on Vancouver Island 2075 Tryon Road Sidney, BC V8L 3X9 Ph: 250.656.2832 Fax: 250.655.1981 E-mail. Categories; Map It; Top Categories ...
Thunderbird Yacht Sales is located in Metro Vancouver of British Columbia state. On the street of Marine Drive and street number is 5776. To communicate or ask something with the place, the Phone number is (604) 921-7457.
3 reviews of THUNDERBIRD MARINA "I have moored my yacht at Thunderbird for many years. I have always found them honest and reliable. ... 5776 Marine Drive. West Vancouver, BC V7W 2S2. Get directions. Recommended Reviews. ... Windward Yacht Sales. 2. Boat Repair. M & P Yacht Centre. 1.
Thunderbird Marina, West Vancouver, British Columbia, V7W 2S2, Canada. Thunderbird Marine Corp. is perhaps the most established of any boating company in Western Canada. Founded in 1952 Thunderbird Yacht Sales has full service yacht brokers and associate broker centres. Clear Filter Owner: broker-thunderbird-yacht-sales-15619.
Thunderbird Yacht Sales Thunderbird Marina . 5776 Marine Drive . West Vancouver, British Columbia, V7W 2S2 Canada 604-305-1795. View Seller Inventory Call Now 604-305-1795 Send Email Request Information. ... West Vancouver, British Columbia, Canada: Measurements LOA: 24 ft: Beam: 8 ft 6 in: Max Draft:
Thunderbird Yacht Sales. West Vancouver at Thunderbird Marina (604) 921-7457 Port Moody at Reed Point Marina (604) 939-0499 ... 5776 Marine Drive West Vancouver, British Columbia Canada V7W 2S2 +1 (604) 921 7457 +1 (604) 921 7486. [email protected]. Sidney Yacht Sales
Vancouver Yacht Sales @ Thunderbird Marina 5776 Marine Drive West Vancouver, British Columbia Canada V7W 2S2 +1 (604) 921 7457 ... Sidney Yacht Sales @ Westport Marina 2075 Tryon Road Sidney, British Columbia Canada V8L 3X9 +1 (250) 656 5832 +1 (250) 655 1981. [email protected]. Port Moody Yacht Sales @ Reed Point Marina 850 Barnet ...
Thunderbird Marina - 5776 Marine Dr, West Vancouver, BC Located at 5776 Marine Dr near you, Thunderbird Marina is a local business within the marinas category of Canpages website. Phone 604-921-7434 to get in contact with Thunderbird Marina that is located in your neighbourhood.
Single Family (freehold) house 3 bedrooms, 3 bathrooms, 5679 marine drive west vancouver, british columbia, for sale $2,295,000. We use cookies and other technologies for functionality, security, and to provide you with a personalized experience on our online services.
Vancouver Yacht Sales @ Thunderbird Marina 5776 Marine Drive West Vancouver, British Columbia Canada V7W 2S2 +1 (604) 921 7457 ... Sidney Yacht Sales @ Westport Marina 2075 Tryon Road Sidney, British Columbia Canada V8L 3X9 +1 (250) 656 5832 +1 (250) 655 1981. [email protected]. Port Moody Yacht Sales @ Reed Point Marina 850 Barnet ...
Vancouver Yacht Sales @ Thunderbird Marina 5776 Marine Drive West Vancouver, British Columbia Canada V7W 2S2 +1 (604) 921 7457 ... Sidney Yacht Sales @ Westport Marina 2075 Tryon Road Sidney, British Columbia Canada V8L 3X9 +1 (250) 656 5832 +1 (250) 655 1981. [email protected]. Port Moody Yacht Sales @ Reed Point Marina 850 Barnet ...
Thunderbird Yacht Sales. West Vancouver at Thunderbird Marina (604) 921-7457 Port Moody at Reed Point Marina (604) 939-0499 ... 5776 Marine Drive West Vancouver, British Columbia Canada V7W 2S2 +1 (604) 921 7457 +1 (604) 921 7486. [email protected]. Sidney Yacht Sales